Special Poster Session 51st International Society for the Study of the Lumbar Spine Annual Meeting 2025
Inflammatory phenotypes in non-specific chronic low back pain (#SP-4f)
INTRODUCTION
Chronic low back pain (CLBP) associated with axial spondyloarthritis (axSpA), an autoimmune condition associated with spinal inflammation, is characterized by morning stiffness, improvement with exercise, worsening with rest, and night-time pain1. These symptoms are measured with questionnaires that assign a binary result (Yes/No) to each symptom, and a diagnosis of inflammatory low back pain (IBP) assigned based on the number of Yes responses. While it is a hallmark of axSpA, IBP is also common in subjects with non-specific CLBP (nsCLBP)2. The current IBP criteria, developed for use in axSpA, may not capture clinically relevant heterogeneity of inflammatory symptoms in nsCLBP. The objective of this study was to determine if subgroups of patients exist based on the distribution of these symptoms, indicating distinct IBP phenotypes within nsCLBP.
METHODS
BackHome is an online study conducted using web-and mobile based recruitment, enrollment, consent, and participation [ref]. All participants meet the criteria for cLBP as defined by NIH Pain Consortium Research Task Force (RTF)3. Exclusion criteria are neurologic deficits, fractures, cancer, pregnancy, and autoimmune disorders. This analysis uses baseline data only. Inflammatory symptoms (morning stiffness > 30 minutes, improved pain with exercise, worsened pain with rest, and night-time pain) were scored as Yes/No using a validated questionnaire, creating a vector of multivariate binary data. Finite mixture modeling (FMM) was applied to identify subgroups based on IBP symptoms. The optimal number of clusters was determined by assessing model fit using Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). We examined associations between subgroups and clinical features to explore predictors of cluster membership.
RESULTS
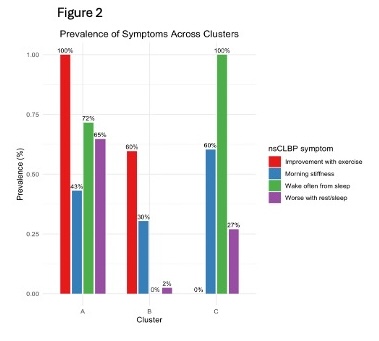
Data from 1,375 participants was analyzed. Inflammatory symptoms were common: morning stiffness 39.1%, improvement with exercise 63.8%, waking from sleep 38.4%, and worsening with rest 26.5%. A 3-cluster solution was the best fit (Figure 1). Symptom distributions across clusters (Figure 2) revealed distinct patterns:
- Cluster A (n=448): High "improvement with exercise" (100%), "wake from sleep" (72%), and "worse with rest" (65%), with moderate "morning stiffness" (43%), suggesting activity-responsive pain with sleep disruptions.
- Cluster B (n=720): Moderate "improvement with exercise" (60%) and "morning stiffness" (30%), but no "wake from sleep" (0%) and minimal "worse with rest" (2%), indicating a mixed symptom profile.
- Cluster C (n=207): High "wake from sleep" (100%) and "morning stiffness" (60%), moderate "worse with rest" (27%), and no "improvement with exercise" (0%), representing severe sleep disruption and rest-related pain without exercise relief.
DISCUSSION
The key finding from this exploratory analysis is that subgroups of patients may exist based on the distribution of inflammatory symptoms, suggesting distinct IBP phenotypes within nsCLBP. This novel finding may have implications for identifying clinically relevant nsCLBP subgroups. Future research should focus on more granular measurement of inflammatory symptoms, given the likely information loss with the current binary classification, evaluating cluster stability in other samples, correlation with imaging and other inflammatory biomarkers, and ultimately clinical trials to determine if clusters predict differential treatment effects.
Supported by the National Institute Of Arthritis And Musculoskeletal And Skin Diseases of NIH; Award Number U19AR076737

- Rudwaleit M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009 Jun;68(6):777-83. PMID: 19297344.
- Weisman MH, et al. The prevalence of inflammatory back pain: population-based estimates from the US National Health and Nutrition Examination Survey, 2009-10. Ann Rheum Dis. 2013 Mar;72(3):369-73. PMID: 22791746
- Deyo R, et al. Focus article: report of the NIH task force on research standards for chronic low back pain. Eur Spine J. 2014. 23:2028-2045. doi: 10.1007/s00586-014-3540-3