Special Poster Session 51st International Society for the Study of the Lumbar Spine Annual Meeting 2025
Tie2-enhanced nucleus pulposus cell-derived extracellular vesicles increase degenerative disc cell proliferation, ameliorate senescence, and present distinct proteomic signatures compared to mesenchymal stromal cell-derived extracellular vesicles (#SP-1b)
INTRODUCTION
Intervertebral disc degeneration (IDD) is a progressive condition affecting all disc tissues. We have recently demonstrated that Tie2-enhanced nucleus pulposus cell-derived extracellular vesicles (NPCTie2+-EVs) significantly outperformed bone marrow mesenchymal stromal cell-derived EVs (BM-MSC-EVs) in mitigating IDD and preserving disc tissue integrity1. However, the mechanisms underlying these superior outcomes remain unclear. This study aimed to characterize the protein content and assess the effect of these EVs on all disc cell types, including annulus fibrosus cells (AFCs) and cartilaginous endplate cells (CEPCs), compared to BM-MSCs-EVs.
METHODS
NPCs (n=10) were isolated from young donors and cultured under Tie2-enhancing conditions2. BM-MSCs (n=4) were sourced commercially or from iliac crest samples during spine surgeries. EVs were isolated from both cell sources through ultracentrifugation and characterized using particle size analysis (qNano), Western blot for EV marker expression (CD63, CD9, Tsg101, Lamin A/C), and transmission electron microscopy (TEM). Degenerative NPCs (dNPCs), AFCs, and CEPCs were isolated from surgical specimens of IDD patients, and treated with NPCsTie2+-EVs or BM-MSC-EVs±10 ng/mL IL-1β, or 10 ng/mL IL-1β alone. EV uptake was assessed using PKH26 staining and confocal microscopy. Cell proliferation was assessed at 3 and 7 days of culture. Cell senescence was evaluated after 3 days of treatment through senescence-associated β-galactosidase (SA-β-Gal) staining. Proteomic hits were analyzed to identify differentially expressed proteins, employing data imputation, fold change (FC) thresholds, and false discovery rate (FDR) adjustments (FDR<0.05). Results were visualized through volcano plots and heatmaps, and significant up- and down-regulated gene hits were analyzed for pathways and processes targeted.
RESULTS
Isolated EVs exhibited typical exosomal characteristics in terms of size, marker expression, and morphology (Fig. 1). Following PKH26 staining, both NPCsTie2+-EVs and BM-MSC-EVs were successfully taken up by dNPCs, AFCs, and CEPCs. NPCTie2+-EVs significantly increased dNPC, AFC, and CEPC proliferation (Fig. 2A) and reduced cell senescence (Fig. 2B) compared to both the controls and cells treated with BM-MSC-EVs, even in the presence of IL-1β. 172 proteins were significantly upregulated, while 303 proteins showed significant downregulation. The three most downregulated proteins were Desmoglein-2 (FC<0.01), Extracellular Superoxide Dismutase (SOD3; FC=0.01), and Melanotransferrin (FC=0.01). In contrast, the top three upregulated proteins included MAP1LC3B2 (FC=71.25), Transmembrane Protein 119 (FC=53.69), and FAM177A1 (FC=47.58). Gene Ontology (GO) analysis revealed that upregulated proteins were strongly associated with wound healing, focal adhesion, and cadherin binding.
DISCUSSION
This study demonstrates that EVs derived from Tie2-enhanced NPCs can significantly stimulate the proliferation and reduce the senescence of degenerative cells isolated from all disc tissues. The observed effects were more pronounced than those of BM-MSC-EVs, suggesting the potential advantages of using tissue-specific progenitor cells as an EV source for IDD treatment. This may be partly explained by the different protein cargo, which may involve distinct signaling pathways related to cell-cell and cell-matrix interactions, warranting further exploration.
Fig. 1. Characterization experiments of isolated EVs.
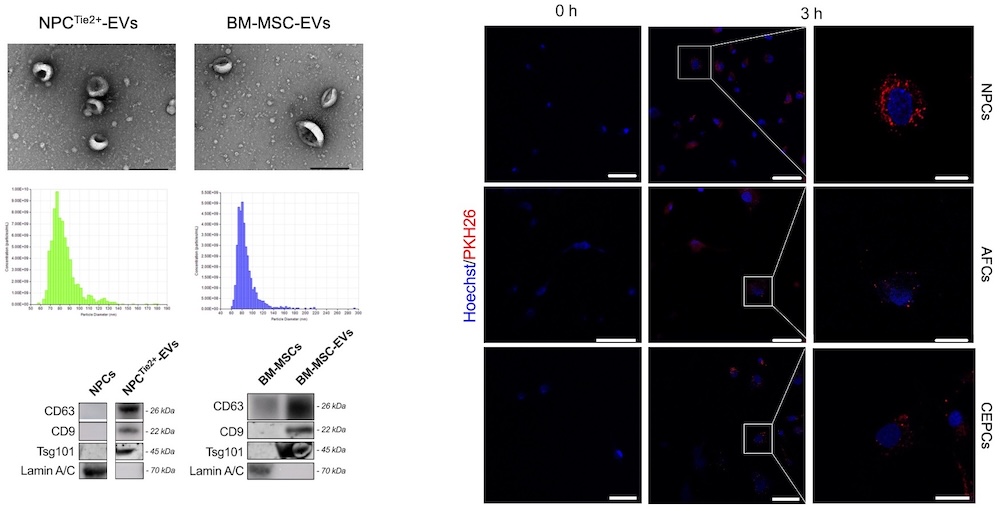
Fig. 2. (A) Cell proliferation and (B) senescence after treatment with NPCTie2+-EVs and BM-MSC-EVs with and without IL-1β.

Fig. 3. Volcano plot overview (A), heatmap of differentially expressed proteins (B), and GO-term analysis of protein hits (C).

- Ambrosio L, Schol J, Ruiz-Fernandez C, Tamagawa S, Soma H, Tilotta V, Di Giacomo G, Cicione C, Nakayama S, Kamiya K, Papalia R, Sato M, Vadalà G, Watanabe M, Denaro V, Sakai D. ISSLS PRIZE in Basic Science 2024: superiority of nucleus pulposus cell- versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain. Eur Spine J. 2024 May;33(5):1713-1727.
- Sako K, Sakai D, Nakamura Y, Schol J, Matsushita E, Warita T, Horikita N, Sato M, Watanabe M. Effect of Whole Tissue Culture and Basic Fibroblast Growth Factor on Maintenance of Tie2 Molecule Expression in Human Nucleus Pulposus Cells. Int J Mol Sci. 2021 Apr 29;22(9):4723.